Dragon makes use of some atomic properties as the atomic weighting schemes for molecular descriptor calculation. In order to select the weightings, click 'General settings' in the 'Settings' menu of the main menu bar. Then, in the tab 'Calculation' select the check boxes corresponding to the weightings to be used in descriptor calculation.
Note that:
| 1. | the number of available molecular descriptors depends on the selected weighting schemes. Only the molecular descriptors that are based on the selected weighting schemes will be enabled in the window 'Select descriptors'. |
| 2. | the list of selected weightings is stored and will be used in successive work sessions of Dragon. |
| 3. | after each selection of weighting schemes, a new work session starts and previous results will be canceled. |
Atomic properties used as the atomic weightings for descriptor calculation in Dragon are:
| ▪ | atomic mass (m); |
| ▪ | van der Waals volume (v); |
| ▪ | atom electronegativity (e); |
| ▪ | atom polarizability (p); |
| ▪ | ionization potential (i); |
| ▪ | intrinsic state (s); |
For each property, the unscaled and carbon atom scaled values can be seen in the window 'Weighting schemes' of the menu 'View' in the main menu bar.
For some molecular descriptors such as the WHIMs, another atomic weighting is derived from the electrotopological state indices of Kier and Hall (Kier, L.B., Hall, L.H. (1990), Pharm.Res., 7, 801-807). These are atomic indices calculated as:
In addition to the basic physico-chemical properties of atoms, Dragon implements calculation of molecular descriptors that are based on the intrinsic state. The intrinsic state (I-state) of the ith atom is a local vertex invariant calculated from the molecular graph as the following:
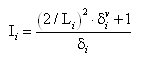
where L is the principal quantum number, δv is the number of valence electrons (valence vertex degree) and δ is the number of sigma electrons (simple vertex degree) of the ith atom in the H-depleted molecular structure. Since the I-state is only defined for non-H atoms, Dragon adopts the convention to use the value of 1 for hydrogens.
Data are taken from CRC Handbook of Chemistry and Physics by D.R. Lide (editor), CRC press 2009-2010, 90th edition.
Atomic No. (Z) |
Atom |
Mass (m) |
Van der Waals volume (v) |
Sanderson electroneg. (e) |
Polarizability (p) |
Ionization potential (i) |
1 |
H |
1.01 |
5.42 |
2.59 |
0.67 |
13.5984 |
2 |
He |
4.003 |
11.49 |
n.a. |
0.2 |
24.5874 |
3 |
Li |
6.941 |
25.25 |
0.89 |
24.3 |
5.3917 |
4 |
Be |
9.012 |
n.a. |
1.81 |
5.6 |
9.3226 |
5 |
B |
10.81 |
n.a. |
2.28 |
3.03 |
8.298 |
6 |
C |
12.01 |
20.58 |
2.75 |
1.76 |
11.2603 |
7 |
N |
14.01 |
15.6 |
3.19 |
1.1 |
14.5341 |
8 |
O |
16 |
14.71 |
3.65 |
0.8 |
13.6181 |
9 |
F |
19 |
13.31 |
4 |
0.56 |
17.4228 |
10 |
Ne |
20.18 |
15.3 |
4.5 |
0.39 |
21.5645 |
11 |
Na |
22.991 |
49 |
0.56 |
23.6 |
5.1391 |
12 |
Mg |
24.305 |
21.69 |
1.32 |
10.6 |
7.6462 |
13 |
Al |
26.98 |
n.a. |
1.71 |
6.8 |
5.9858 |
14 |
Si |
28.09 |
38.79 |
2.14 |
5.38 |
8.1517 |
15 |
P |
30.97 |
24.43 |
2.52 |
3.63 |
10.4867 |
16 |
S |
32.07 |
24.43 |
2.96 |
2.9 |
10.36 |
17 |
Cl |
35.45 |
22.45 |
3.48 |
2.18 |
12.9676 |
18 |
Ar |
39.948 |
27.83 |
3.31 |
1.64 |
15.7596 |
19 |
K |
39.098 |
87.11 |
0.45 |
43.4 |
4.3407 |
20 |
Ca |
40.078 |
n.a. |
0.95 |
22.8 |
6.1132 |
21 |
Sc |
44.956 |
n.a. |
1.02 |
17.8 |
6.5615 |
22 |
Ti |
47.867 |
n.a. |
1.09 |
14.6 |
6.8281 |
23 |
V |
50.942 |
n.a. |
1.39 |
12.4 |
6.7462 |
24 |
Cr |
52 |
n.a. |
1.66 |
11.6 |
6.7665 |
25 |
Mn |
54.94 |
n.a. |
2.2 |
9.4 |
7.434 |
26 |
Fe |
55.85 |
n.a. |
2.2 |
8.4 |
7.9024 |
27 |
Co |
58.93 |
n.a. |
2.56 |
7.5 |
7.881 |
28 |
Ni |
58.69 |
18.14 |
1.94 |
6.8 |
7.6398 |
29 |
Cu |
63.55 |
11.49 |
1.98 |
6.1 |
7.7264 |
30 |
Zn |
65.39 |
11.25 |
2.23 |
7.1 |
9.3942 |
31 |
Ga |
69.72 |
27.39 |
2.42 |
8.12 |
5.9993 |
32 |
Ge |
72.61 |
n.a. |
2.62 |
6.07 |
7.9 |
33 |
As |
74.92 |
26.52 |
2.82 |
4.31 |
9.8152 |
34 |
Se |
78.96 |
28.73 |
3.01 |
3.77 |
9.7524 |
35 |
Br |
79.9 |
26.52 |
3.22 |
3.05 |
11.8138 |
36 |
Kr |
83.8 |
34.53 |
2.91 |
2.48 |
13.9996 |
37 |
Rb |
85.468 |
n.a. |
0.31 |
47.3 |
4.1771 |
38 |
Sr |
87.62 |
n.a. |
0.72 |
27.6 |
5.6949 |
39 |
Y |
88.906 |
n.a. |
0.65 |
22.7 |
6.2173 |
40 |
Zr |
91.224 |
n.a. |
0.9 |
17.9 |
6.6339 |
41 |
Nb |
92.906 |
n.a. |
1.42 |
15.7 |
6.7589 |
42 |
Mo |
95.94 |
n.a. |
1.15 |
12.8 |
7.0924 |
43 |
Tc |
98 |
n.a. |
n.a. |
11.4 |
7.28 |
44 |
Ru |
101.07 |
n.a. |
n.a. |
9.6 |
7.3605 |
45 |
Rh |
102.906 |
n.a. |
n.a. |
8.6 |
7.4589 |
46 |
Pd |
106.42 |
18.14 |
n.a. |
4.8 |
8.3369 |
47 |
Ag |
107.87 |
21.31 |
1.83 |
7.2 |
7.5762 |
48 |
Cd |
112.41 |
16.52 |
1.98 |
7.2 |
8.9938 |
49 |
In |
114.82 |
30.11 |
2.14 |
10.2 |
5.7864 |
50 |
Sn |
118.71 |
42.8 |
2.3 |
7.7 |
7.3438 |
51 |
Sb |
121.76 |
n.a. |
2.46 |
6.6 |
8.6084 |
52 |
Te |
127.6 |
36.62 |
2.62 |
5.5 |
9.0096 |
53 |
I |
126.9 |
32.52 |
2.78 |
5.35 |
10.4513 |
54 |
Xe |
131.29 |
42.21 |
2.34 |
4.04 |
12.1298 |
55 |
Cs |
132.905 |
n.a. |
0.22 |
59.6 |
3.8939 |
56 |
Ba |
137.327 |
n.a. |
0.68 |
39.7 |
5.2117 |
57 |
La |
138.906 |
n.a. |
n.a. |
31.1 |
5.5769 |
58 |
Ce |
140.116 |
n.a. |
n.a. |
29.6 |
5.5387 |
59 |
Pr |
140.908 |
n.a. |
n.a. |
28.2 |
5.473 |
60 |
Nd |
144.24 |
n.a. |
n.a. |
31.4 |
5.525 |
61 |
Pm |
145 |
n.a. |
n.a. |
30.1 |
5.55 |
62 |
Sm |
150.36 |
n.a. |
n.a. |
28.8 |
5.6437 |
63 |
Eu |
151.964 |
n.a. |
n.a. |
27.7 |
5.6704 |
64 |
Gd |
157.25 |
n.a. |
n.a. |
23.5 |
6.1498 |
65 |
Tb |
158.925 |
n.a. |
n.a. |
25.5 |
5.8639 |
66 |
Dy |
162.5 |
n.a. |
n.a. |
24.5 |
5.9389 |
67 |
Ho |
164.93 |
n.a. |
n.a. |
23.6 |
6.0215 |
68 |
Er |
167.26 |
n.a. |
n.a. |
22.7 |
6.1077 |
69 |
Tm |
168.934 |
n.a. |
n.a. |
21.8 |
6.1843 |
70 |
Yb |
173.04 |
n.a. |
n.a. |
21 |
6.2542 |
71 |
Lu |
174.967 |
n.a. |
n.a. |
21.9 |
5.4259 |
72 |
Hf |
178.49 |
n.a. |
n.a. |
16.2 |
6.8251 |
73 |
Ta |
180.948 |
n.a. |
n.a. |
13.1 |
7.5496 |
74 |
W |
183.84 |
n.a. |
0.98 |
11.1 |
7.864 |
75 |
Re |
186.207 |
n.a. |
n.a. |
9.7 |
7.8335 |
76 |
Os |
190.23 |
n.a. |
n.a. |
8.5 |
8.4382 |
77 |
Ir |
192.217 |
n.a. |
n.a. |
7.6 |
8.967 |
78 |
Pt |
195.08 |
21.31 |
n.a. |
6.5 |
8.9588 |
79 |
Au |
196.97 |
19.16 |
n.a. |
5.8 |
9.2255 |
80 |
Hg |
200.59 |
15.6 |
2.2 |
5.7 |
10.4375 |
81 |
Tl |
204.38 |
31.54 |
2.25 |
7.6 |
6.1082 |
82 |
Pb |
207.2 |
34.53 |
2.29 |
6.8 |
7.4166 |
83 |
Bi |
208.98 |
n.a. |
2.34 |
7.4 |
7.2855 |
84 |
Po |
210 |
n.a. |
n.a. |
6.8 |
8.414 |
85 |
At |
210 |
n.a. |
n.a. |
6 |
n.a. |
86 |
Rn |
222 |
n.a. |
n.a. |
5.3 |
10.7485 |
87 |
Fr |
223 |
n.a. |
n.a. |
48.7 |
4.0727 |
88 |
Ra |
226 |
n.a. |
n.a. |
38.3 |
5.2784 |
89 |
Ac |
227 |
n.a. |
n.a. |
32.1 |
5.17 |
90 |
Th |
232.038 |
n.a. |
n.a. |
32.1 |
6.3067 |
91 |
Pa |
231.036 |
n.a. |
n.a. |
25.4 |
5.89 |
92 |
U |
238.029 |
26.95 |
n.a. |
27.4 |
6.1941 |
93 |
Np |
237 |
n.a. |
n.a. |
24.8 |
6.2657 |
94 |
Pu |
244 |
n.a. |
n.a. |
24.5 |
6.026 |
95 |
Am |
243 |
n.a. |
n.a. |
23.3 |
5.9738 |
96 |
Cm |
247 |
n.a. |
n.a. |
23 |
5.9914 |
97 |
Bk |
247 |
n.a. |
n.a. |
22.7 |
6.1979 |
98 |
Cf |
251 |
n.a. |
n.a. |
20.5 |
6.2817 |
99 |
Es |
252 |
n.a. |
n.a. |
19.7 |
6.42 |
100 |
Fm |
257 |
n.a. |
n.a. |
23.8 |
6.5 |
101 |
Md |
258 |
n.a. |
n.a. |
18.2 |
6.58 |
102 |
No |
259 |
n.a. |
n.a. |
17.5 |
6.65 |
103 |
Lr |
262 |
n.a. |
n.a. |
n.a. |
4.9 |
104 |
Rf |
261 |
n.a. |
n.a. |
n.a. |
6 |
105 |
Db |
262 |
n.a. |
n.a. |
n.a. |
n.a. |
106 |
Sg |
266 |
n.a. |
n.a. |
n.a. |
n.a. |
107 |
Bh |
264 |
n.a. |
n.a. |
n.a. |
n.a. |
108 |
Hs |
269 |
n.a. |
n.a. |
n.a. |
n.a. |
109 |
Mt |
268 |
n.a. |
n.a. |
n.a. |
n.a. |
110 |
Ds |
271 |
n.a. |
n.a. |
n.a. |
n.a. |
By clicking the upper right button ![]() in the window 'Weighting schemes' of the 'View' menu, a text file (.txt) can be generated with any name and storage location.
in the window 'Weighting schemes' of the 'View' menu, a text file (.txt) can be generated with any name and storage location.